Crystal Field Theory (CFT)
Crystal Field Theory (CFT): Overview
This topic covers concepts, such as, Crystal Field Splitting Theory, Crystal Field Splitting in Tetrahedral Complexes, Conditions for Formation of Square Planar Complexes & Conditions for Formation of Tetrahedral Complexes etc.
Important Questions on Crystal Field Theory (CFT)
The value of the spin only magnetic moment for one of the following configurations is BM. The correct one is
A complex of metal has the following electronic distribution in orbitals,
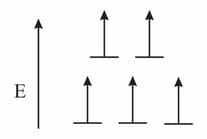
The neutral M has a ground state electronic configuration of Which of the following complexes is consistent with the electronic distribution of (as described above)?
In which of the following complexes, magnetic moment will change when all the ligands are replaced by ion to form a cyano complex?
In Wilkinson's catalyst, the hybridization of central metal ion and its shape are respectively:
An octahedral complex with magnetic moment may have:
(a) configuration with weak field ligand.
(b) configuration with strong field ligand.
(c) configuration with strong field ligand.
(d) configuration with weak field ligand.
Crystal field splitting energies for octahedral and tetrahedral geometries caused by the same ligands are related through the expression
The order of octahedral crystal field energy for orbitals are
According to crystal field theory, total number of unpaired electrons present in the following molecules and will be
For next two question please follow the same
When degenerate -orbitals of an isolated atom/ion come under influence of magnetic field of ligands, the degeneracy is lost. The two set t2g (dxy, dyz,dxz) and eg (dz2, dx2-y2) are either stabilized or destabilized depending upon the nature of magnetic field. It can be expressed diagrammatically as :
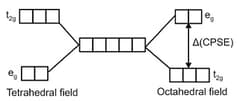
Value of depends upon nature of ligand and a spectrochemical series has been made experimentally, for tetrahedral complexes, is about times to ( for octahedral complex). This energy lies in visible region and i.e., why electronic transition are responsible for colour. Such transitions are not possible with and configuration.
form four complexes with four different ligands which are [Cr(Cl)6]3-, [Cr(H2O)6]3+, [Cr(NH3)6]3+ and [Cr(CN)6]3-. The order of in these complexes is in the order :
Increasing crystal field strength of the different ligands is :
When all the ligands are replaced by ion to form a cyano complex, which of the following complexes show magnetic moment?
forms four complexes with four different ligands which are and . The order of crystal field stabilization energy in these complexes are
Which of the following complex ions has electrons that are symmetrically filled in both and orbitals?
Which of the following statement is incorrect regarding below complexes?
A d-block element forms an octahedral complex, but its magnetic moment remains the same either in strong field or in weak field ligand. Among the following, which is/are correct?
The coordination complex that shows zero crystal field stabilisation energy () is:
Consider the following complex ions -
Give the correct order of crystal field splitting energy.
For the complex, the value of crystal field splitting ( ) is . The crystal field stabilization energy (CFSE) in the complex (in ) would be :
What is the hybridization of in the brown ring formation?
Which of the following is true about ?
